Surface Tension and Cohesion Occur in Pure Water Because Water
This gives water unique properties such as a relatively high boiling point high specific heat cohesion adhesion and density. Put some water in a jar.
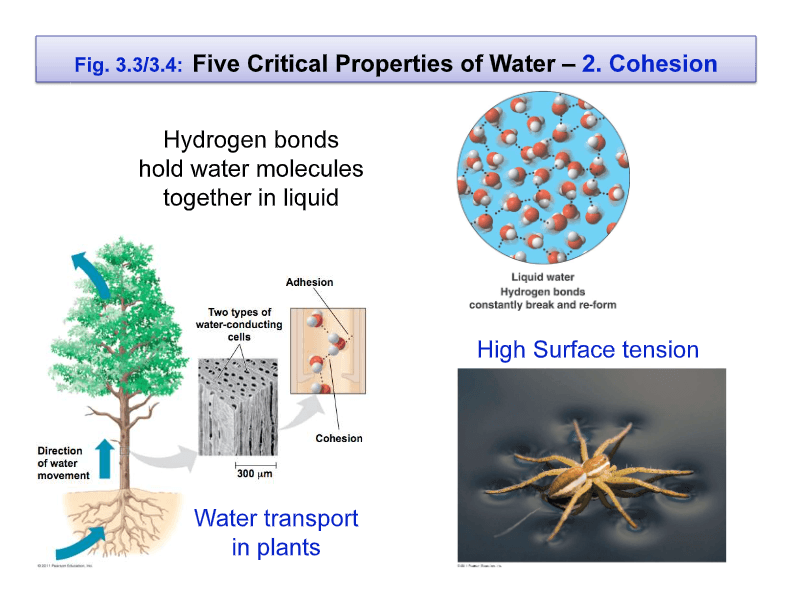
2 2 Water And Hydrogen Bonding Biology Quizizz
Surface tension and cohesion occur in pure water because.

. With water you can think of it as when water sticks to the inside of a glass. Surface tension not only depends upon the forces of attraction between the particles within the given liquid but also on the forces of attraction of solid liquid or gas in contact with it. Because water seems so ubiquitous many people are unaware of the unusual and unique properties of water including.
Why a meniscus occurs Adhesion is responsible for a meniscus and this has to do in part with waters fairly high surface tension. Surface tension and cohesion result from hydrogen bonds between water molecules whereas adhesion results from hydrogen bonds between water and other polar molecules. Surface tension heat of vaporization and vapor pressure.
A hot soup tastes much more delicious than a cold one because the surface tension of a hot soup is lower than that of the cold soup. It turns out that this surface tension is the result of the tendency of water molecules to attract one another. As a result cohesion of the water becomes less and surface tension decreases.
There are three different forms of water or H 2 O. In brief surface tension can be observed due to cohesion. The same argument applies at the surface as in bulk for small solute molecules and ions.
The surface tension allows the liquid to resist the external force of the bug. And therefore it spreads over a larger area of the tongue. Hydrogen bonds tend to pull water molecules at the surface together reducing the curvature of the.
We apply a force to the liquid such that it separates. The partial charges on a water molecule occur because of _____. Cohesion occurs because the water molecules stick to each other.
Cohesive forces are responsible for surface tension a phenomenon that results in the tendency of a liquids surface to resist rupture when placed under tension or stress. Work of Cohesion and Adhesion. When the molecules possess.
It would take a force of 72 dynes to break a surface film of water 1 cm long. Especially when the water is exposed to charged surfaces such. The surface tension arises from the polar nature of the water molecule.
Because the surface tension was stronger in front of the boat the boat was moved forward. This applies to polar solvents and water is both polar and a strong hydrogen-bonding molecule. This in terms means that covering more taste receptors somehow makes the brain interpret the soup as tastier.
Surface tension is a property of a liquid that allows them to resist external forces. The evaporation of water from the surfaces of mesophyll cells causes the airwater interface to retreat into the cellulose matrix of the. Boiling and freezing points.
The surface tension of water is 72 dynescm at 25C. Below infographic summarizes the difference between. The natural form of a water drop occurs during the lowest energy state the state where the atoms in the molecule are using the least amount of energy.
Cohesion causes the surfaces tension of water. Pure water has a pH of 7 but acid rain has a pH close to 3. Which property of water is the result of hydrogen bonds quizlet.
Water molecules are polar so they form hydrogen bonds. Attraction between water molecules and other molecules. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension.
A meniscus is a curve in the surface of a molecular substance water of course when it touches another material. It combines the concepts of cohesion and adhesion. Cohesion occurs because the water molecules stick to each other.
Solid ice liquid water and gas steam. The weakening of surface tension occurs because the soap molecules move between the water molecules and weaken their attraction for one another. Surface tension is an important factor in the phenomenon of capillarity which is an effects effect capillary action that occurs when liquids are.
The unequal sharing of electrons between hydrogen and oxygen. Surface tension is caused by a strong attraction between the molecules cohesion that cause them to link together and remain uniform even when placed on differing surfaces adhesion. The key difference between cohesion and surface tension is that cohesion describes the intermolecular forces occur between identical molecules whereas surface tension describes the property of elasticity of the surface of a liquid.
Surface Tension of Water. Surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid which tends to minimise surface area. What do cohesion surface tension and adhesion have in common with reference to water.
The molecules at the surface of a glass of water do not have other water molecules on all sides of them and consequently they cohere more strongly to those directly associated with them in this case next to and below them but not above. Cohesion and surface tension keep the hydrogen bonds. The ion must have a solvation sheath a coat of solvent molecules to insulate the charge otherwise it wouldnt go into solution.
The surface tension of water decreases significantly with temperature as shown in the graph. Because of the relatively high attraction of water molecules for each other water has a higher surface tension 728 mNm at 20C 68F compared to the surface tension of many other liquids. Cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top.
The work we have done is called the Work of Cohesion. Water has surface tension because molecules on the outside aligned and are held together by the hydrogen bonds. A forms nonpolar bonds B forms ionic bonds C resists the breaking of hydrogen bonds D forms weak bonds E resists the breaking of ionic bonds.
The widely supported cohesiontension theory of water transport explains the importance of a continuous water column and the mechanism of long-distance ascent of sap in plants Dixon 1914 Tyree 2003 Angeles et al. It is defined as the surface tension times the amount of new area created. Why is water the universal solvent.
Lets imagine that we have a tube of water or some other pure liquid whose cross-sectional area is one cm 2. O This attraction is sometimes stronger than waters cohesive forces. The surface tension allows the liquid to resist the external force of the bug.
Water molecules at the surface at the water-air interface will form hydrogen bonds with their neighbors just like water molecules deeper within the liquid. Surface tension most commonly occurs at air-water interfaces where it resists breakage of the surface. Water has surface tension because molecules on the outside aligned and are held together by the.
Water has a high surface tension because of the hydrogen bonding of surface molecules.

Properties Of Water Water H 2 O Oxygen And Hydrogen Are Bonded Together By Covalent Bonds O And H Share Some Electrons Ppt Download

Solved Question 41 Surface Tension And Cohesion Occur In Chegg Com

No comments for "Surface Tension and Cohesion Occur in Pure Water Because Water"
Post a Comment